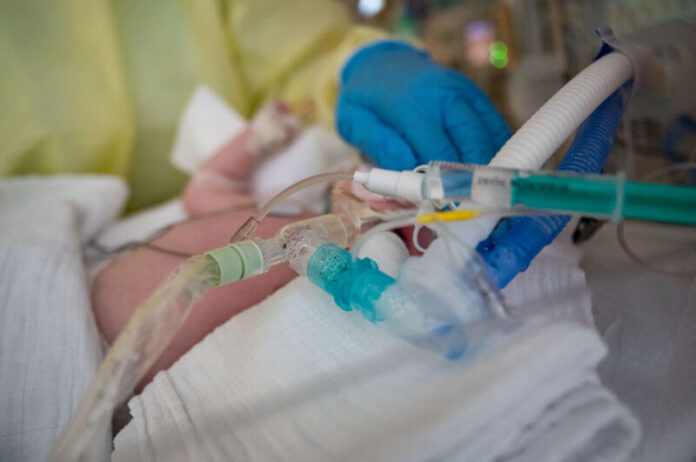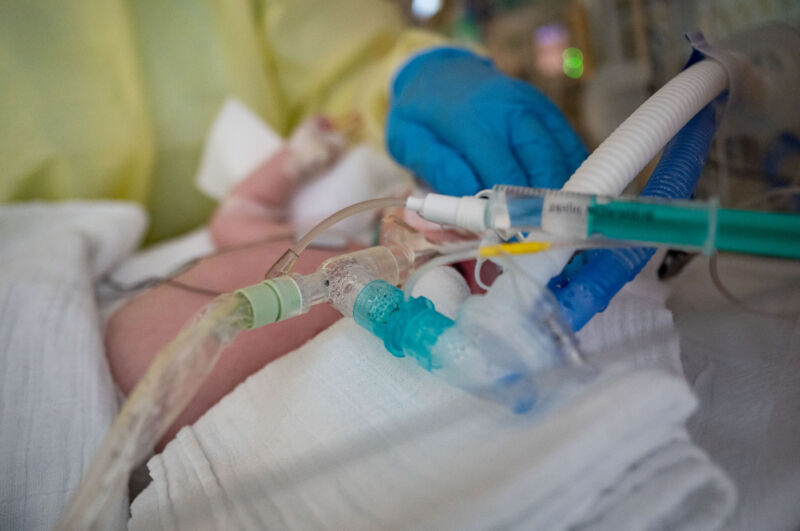
Enlarge / An intensive care nurse cares for a patient suffering from respiratory syncytial virus (RSV), who is being ventilated in the children's intensive care unit of the Olga Hospital of the Stuttgart Clinic in Germany. (credit: Getty | picture alliance)
A Pfizer vaccine designed to protect newborns and infants from severe RSV illness won a recommendation from the Centers for Disease Control and Prevention Friday—but only for seasonal use.
The vaccine is Pfizer’s bivalent RSVpreF vaccine, called Abrysvo, and is administered to pregnant people late in gestation, between 32 and 36 weeks.
RSV, or respiratory syncytial (sin-SISH-uhl) virus, is the leading cause of hospitalization for infants in the US. Each year, 1.5 million children seek out-patient care for RSV, with 58,000 to 80,000 ending up in the hospital and 100 to 300 tragically dying from the infection.
Read 8 remaining paragraphs | Comments
Ars Technica - All contentContinue reading/original-link]