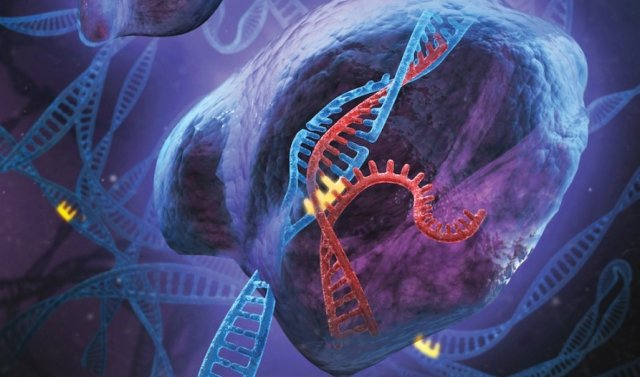
Enlarge / Artist's conception of the CRISPR system in action, with the guide RNA (red) leading a protein to a specific site in the genomic DNA (blue) where it makes a cut. (credit: Stephen Dixon and Feng Zhang)
The UK has become the first country to approve a therapy based on Crispr gene editing, with the regulator authorising a treatment for sickle cell disease and beta thalassaemia.
The Medicines and Healthcare products Regulatory Agency has approved the therapy, called Casgevy, which was developed by Vertex Pharmaceuticals and Crispr Therapeutics. The drug could be used to replace bone marrow transplants.
The UK regulator has promised to focus on speeding the most innovative treatments to market after being given permission from next year to cut its workload by following other countries’ recommendations on approvals of other drugs. It had been struggling to keep up because of a lack of resources after the UK left the EU, where it had been a key part of the bloc’s regulatory agency.
Read 9 remaining paragraphs | Comments
Ars Technica - All contentContinue reading/original-link]